Title: Ammonium salts are a reservoir of nitrogen on a cometary nucleus and possibly on some asteroids
Authors: Olivier Poch et al.
First Author’s Institution: Université Grenoble Alpes, Centre National de la Recherche Scientifique (CNRS), Institut de Planétologie et d’Astrophysique de Grenoble (IPAG), 38000 Grenoble, France.
Status: Published in Science
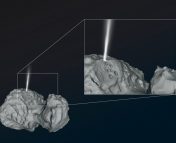
Comets preserve information on the earliest stages of solar system formation and on the composition of its building blocks. Major advancements in our understanding of these planetary bodies have happened thanks to the Rosetta spacecraft, which gained worldwide fame for visiting the Jupiter-family comet, Comet 67-P back in 2014. One of the earliest science results of the mission came from its dust-mass spectrometer, the COSIMA instrument, which collected dust grains originating from the material blasting off of the comet’s surface, and measured their composition. COSIMA measured an average nitrogen-to-carbon ratio, or N/C ratio, of the dust grains to be 0.035 +/- 0.011. This value is much lower than the solar N/C value, which is 0.29 +/- 0.12. Since comets formed very early in the history of solar system (~ 4.5 billion years ago) and have undergone minimal chemical changes since then, we expect their elemental composition to be similar to the nebula that also formed the Sun. So, why was 67P’s nitrogen content found to be lower? Is there an unknown reservoir of nitrogen in comets?
Enter today’s paper..
The mysterious infrared absorber on comet 67P
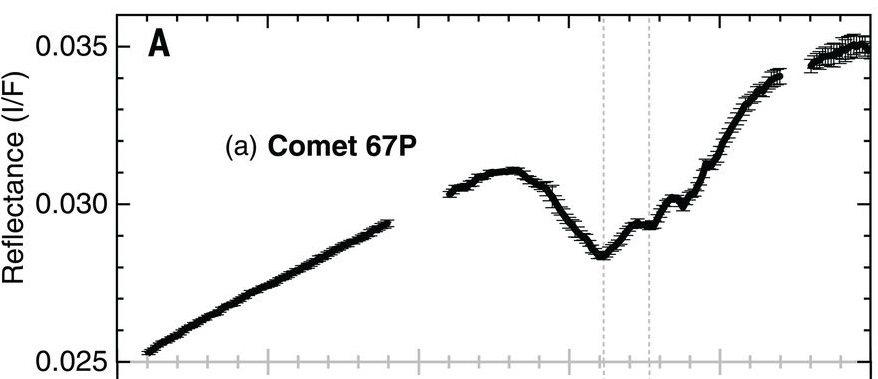
Reflectance spectroscopy is the most popular remote sensing tool that is used to decipher the composition of surfaces of solar system bodies. When sunlight falls on a planetary body’s surface, it gets scattered and reflected away governed by the physical and chemical properties of the surface. The spectrum of this reflected light holds the signatures or ‘fingerprints’ of various species that make up the material on the planetary body’s surface. Analyzing this reflected light was exactly the job of the VIRTIS-M instrument on the Rosetta spacecraft. VIRTIS-M, which stands for the Visible and InfraRed Thermal Imaging Spectrometer Mapping Channel Instrument (I’m pretty sure the acronym came first in this case), mapped the comet extensively in 2014 and 2015. Comet 67P was found to be spectrally very uniform, with most areas having very low reflectance (comets are usually darker than charcoal) and a gentle positive slope in the visible and near-infrared regions (~ 0.4-2.5 microns). These two features have been attributed to non-volatile organics mixed with opaque minerals. However, a broad absorption feature from 2.8 to 3.6 microns, centered at 3.2 microns, was also persistently observed in the reflectance data over the course of the mission (Figure 1). Since the data was first analyzed in 2015, there were speculations about functional groups like carboxyl (-COOH) and ammonium (NH
) being plausible candidates for this absorption feature. However, a lack of reference spectral data for these compounds had prevented a firm attribution of this feature.
Spectral identification of ammonium salts
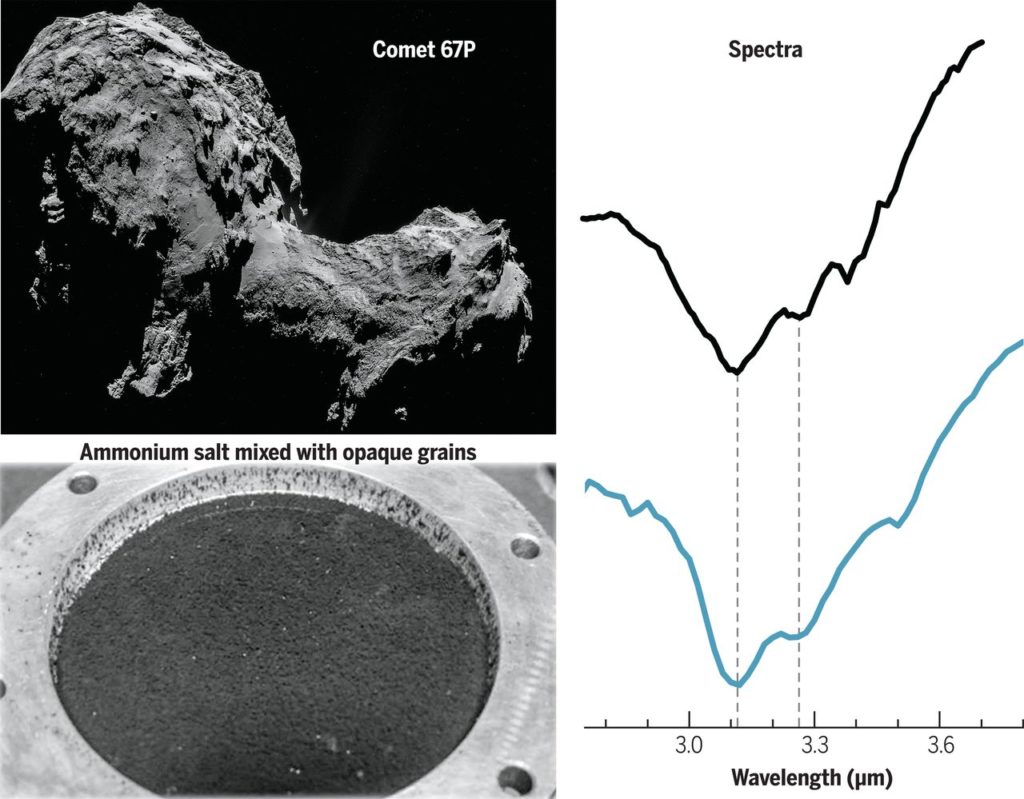
The authors of today’s paper conducted laboratory experiments to produce analogs of cometary surface material and measured their reflectance spectra under comet-like conditions of low temperature and high vacuum (see Figure 2). These analog materials were mostly mixtures of opaque grains and a set of trial compounds, taken one at a time, to figure out which one produces the 3.1 and 3.2 microns absorption bands as seen in Figure 1. A thorough investigation – spanning salts, water ice grains, carboxylic acid and hydrated minerals – revealed that ammonium ( NH ) salts produce the absorption bands at the right wavelengths and in the right shapes! Although the counter-ion for the salt (the negative ion), could not be constrained, the authors conclude formate ( HCOO
) is the most likely candidate, given that formic acid (HCOOH) was directly detected by Rosetta’s mass spectrometer ROSINA which analyzed the composition of the comet’s gaseous atmosphere. Hence, the authors’ most favored candidate for the mystery absorption feature in the 3 microns region of Comet 67 P’s reflectance spectrum is ammonium formate (NH
HCOO
), whose spectrum is shown in Figure 2.
The nitrogen budget of comet 67P
So how does this new discovery relate to the discrepancy between the comet’s N/C ratio and the solar N/C ratio? The authors say that COSIMA, Rosetta’s mass spectrometer that analyzed the dust grains coming off of the comet, probably did not detect ammonium salts because they would have sublimated during the multiple-day-long pre-analysis storage of the particles. Hence, the nitrogen in the ammonium salts went unaccounted for, lowering the overall N/C ratio that was measured by the COSIMA. The authors propose that ammonium salts may constitute a substantial reservoir of nitrogen in comet 67P, and possibly other comets and small bodies, and bring their N/C ratio value closer to the solar value.
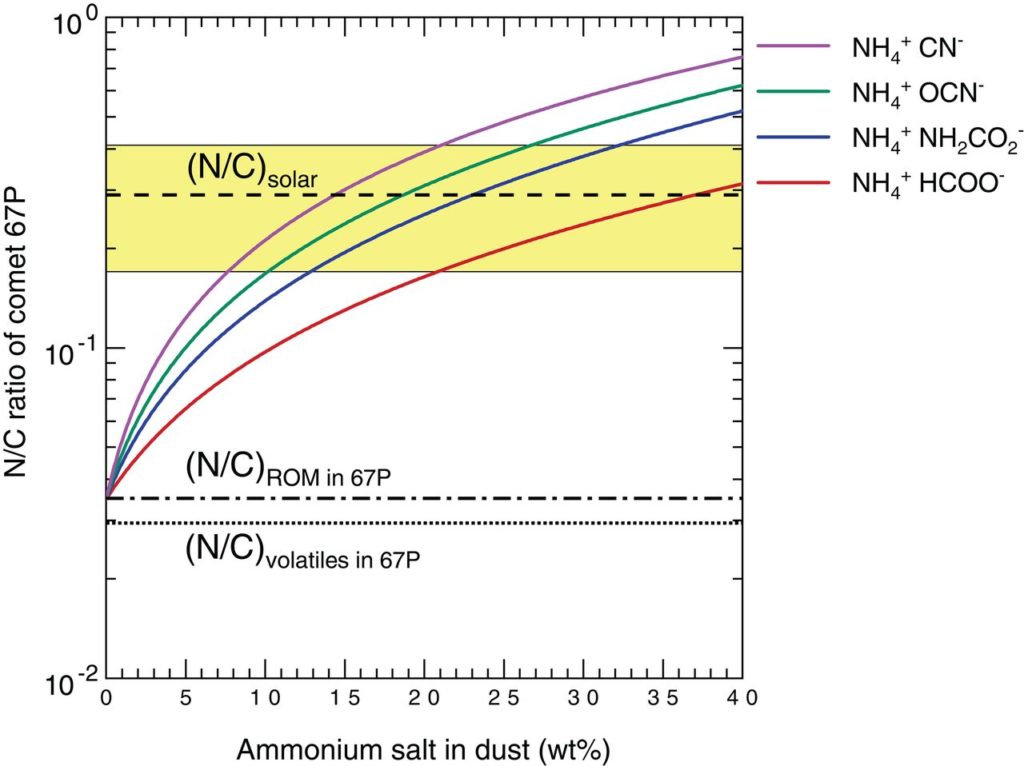
The depth of the reflectance spectrum’s absorption band, in the 3 microns region, also led the authors to constrain the amount of ammonium salts on the comet’s surface at an upper limit of 40%-by-weight. As shown in Figure 3, a 10-30%-by-weight reservoir of ammonium salts on the comet, which would account for more nitrogen than what’s available in the non-volatile organics and volatile species like NH3 and N2, raises the N/C ratio of comet 67P to be consistent with the solar value.
Broader implications
Several asteroids in the Main Belt, Jupiter’s Trojan asteroids, and its small moon Himalia have similar spectra to that of comet 67P, with a broad absorption feature at 3.1 to 3.2 microns, which could also be due to ammonium salts. The dwarf planet Ceres has ammoniated phyllosilicates on its surface, which may have formed from ammonium ions inherited from outer solar system objects with compositions similar to that of comet 67P. If ammonium salts were also present in sufficient abundance in planetesimals during the early Solar System, they would have provided a solid form of nitrogen closer to the Sun than the more volatile N2 and NH3 ices and therefore be available for planetary accretion.