Title: An ALMA Survey of H2CO in Protoplanetary Disks
Authors: Jamila Pegues, Karin Oberg, J. Bergner et al.
First Author Institution: Center for Astrophysics | Harvard & Smithsonian
Open access arXiv closed access on ApJ
Person. Woman. Man. Camera. TV. What do all of these have in common? They are complex organisms or objects which are made of organic molecules. If we want to understand the origins of such earthly things, we need to understand how and where they form within protoplanetary disks. H2CO, or formaldehyde, is one of the most abundant organic molecules in the universe, and can serve as a precursor to more complex organic molecules. Observing the location at which H2CO resides within a disk will shed insight into its formation, and thus the protoplanetary disk’s ability to form more complex molecules. Today’s paper surveys 15 protoplanetary disks looking at multiple H2CO lines. The authors seek to uncover the temperature, density, and origin of H2CO which will inform our knowledge of chemistry within disks.
How do you form molecules in space?
There are two main formation pathways to form molecules within protoplanetary disks. You can form molecules in the gas via which gas-phase chemistry, or you can form molecules on ice grains via ice-grain chemistry. Astronomers have seen that it is possible to create H2CO in both the gas phase and on grains, however we don’t know what the most common formation mechanism might be within disks until we observe them. If H2CO is predominantly formed in the gas phase, we would expect to see a significant amount of H2CO near the center of a disk. This is because the inner few AU is where the gas is most dense, thus there is a higher likelihood of collisions between molecules that can form H2CO. If, on the other hand, ice-grain chemistry is the most common way to form H2CO, then we would expect to see a ring-like structure when observing the H2CO within a disk. The reason behind this is the fact that molecules ‘freeze-out’ onto grains at a certain temperature. To form H2CO on a dust grain, we need the molecule CO (carbon monoxide) to be chillin’ out on the dust grains. Chuck some Hydrogen at it until it becomes H2CO, then it can be released off the grain for us to observe. So! We need CO to not be in the gas, but to be on the grains available for hydrogens to be thrown at them. That will only happen when the environment is so cold that CO begins to ‘freeze’ onto dust grains. That isn’t going to occur until pretty far away from the central star. That freeze-out location is called a snowline. Thus, if CO needs to be stuck onto ice grains to form H2CO, then we’d only see H2CO near the CO snowline, which will look like a ring around the star.
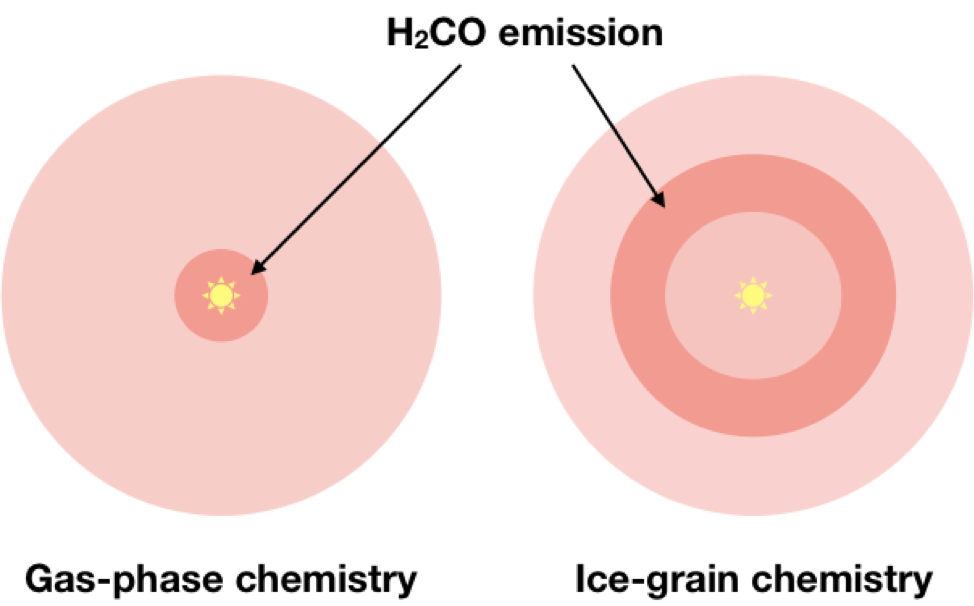
mechanism was gas-phase chemistry (left) or ice-grain chemistry (right)
¿Por qué no los dos?
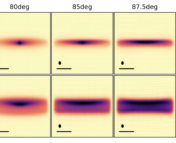
After observing H2CO towards 15 disks, what did these authors find? They found that 8 out of 15 observed disks had H2CO that peaked in the center, 3 had little ‘dips’ (so there was not a super significant amount of H2CO), and two had no H2CO flux in the center. At the same time, they found that 6 out of 15 of their disks had ring-like structures, or plateaus of flux continuing from the center of the disk.
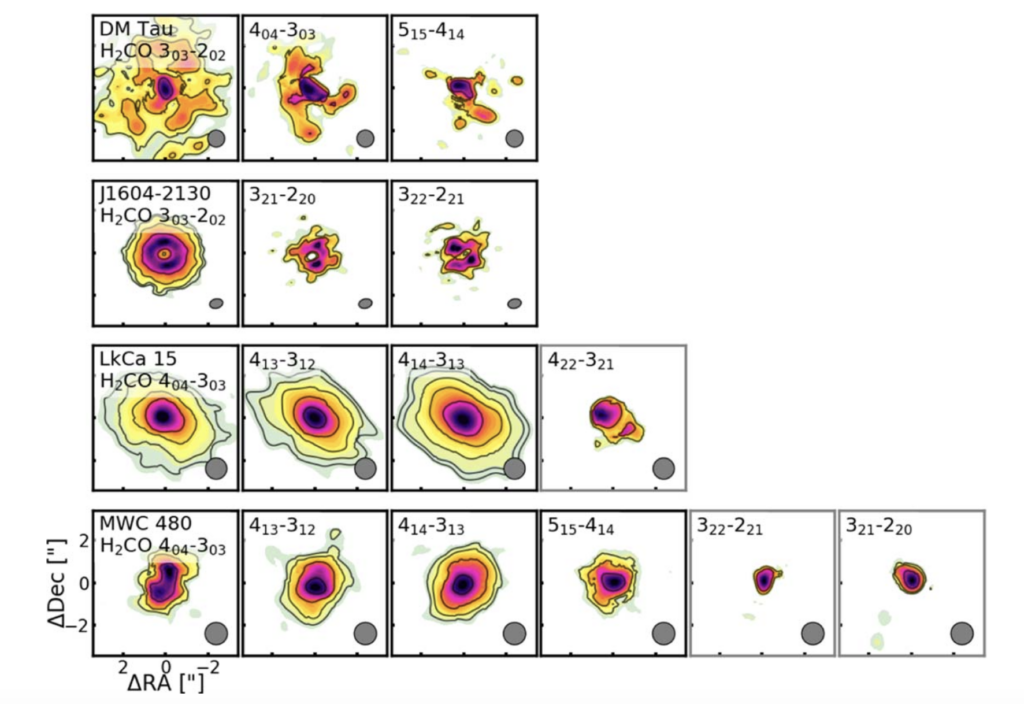
The authors determine that the observed structures are likely formed due to the chemistry that forms H2CO, as opposed to pesky dust getting in the way of seeing the real structure, or old H2CO that might have stuck around from a time before the formation of the protoplanetary disk. From this, they conclude that BOTH gas-phase and ice-grain chemistry are actively forming H2CO within disks.
A Gift That Keeps On Giving
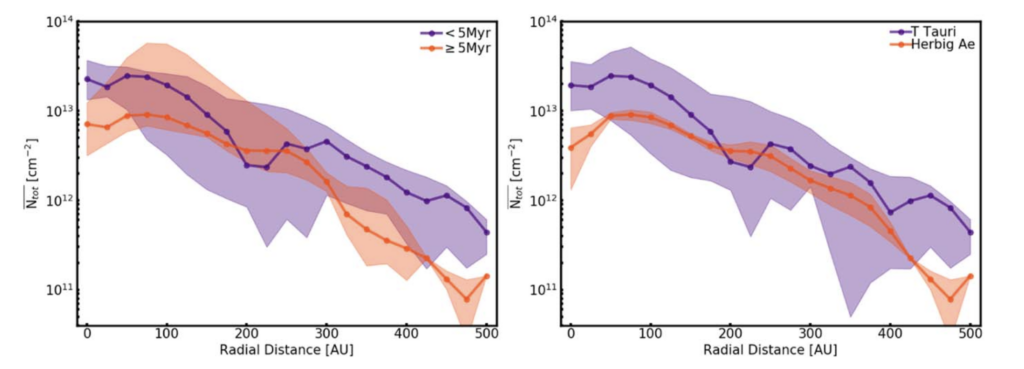
Not only do these observations of H2CO tell us about the formation pathways of this organic molecule, but they can also measure temperature and densities within the disk. For disks with multiple line observations corresponding to different energetic transitions of H2CO, the authors can do some physics magic to pull out the excitation temperature and column densities of H2CO. With that information, they can then compare the densities of H2CO between different types of disks. They see a difference between the biggest and warmest disks (Herbig Ae disks) and the cooler smaller disks (T Tauri disks). The Herbig Ae disks tend to have less H2CO than the lower mass/cooler counterparts. This is consistent with other lower resolution observations of H2CO. This relation is still tentative, as this samples only a handful of disks, but the authors propose this correlation to be due to Herbig Ae disks being too warm for ice-grain chemistry
Further observations of H2CO in more disks, both Herbig and T Tauri, may help shed light on the viability of life around different types of stars. Constraining the abundance and origin of this molecule has provided another vital stepping stone on our way to understanding how complex life forms out of star stuff.