Title: Phosphine gas in the cloud decks of Venus
Authors: Jane S. Greaves, Anita M. S. Richards, William Bains, Paul B. Rimmer et al.
First Author Institution: School of Physics and Astronomy, Cardiff University and Institute of Astronomy, University of Cambridge, Cambridge UK.
Status: Accepted to Nature Astronomy, open access
“The temperatures and pressures from ~40-60 km above the surface of Venus are like Florida, except for the part where the water droplets are highly concentrated sulfuric acid. Venus sucks!”
The Observation of Phosphine
In the summer of 2017, the James Clerk Maxwell Telescope (JCMT) in Hawaii turned towards our sister planet, Venus. The goal of that night was to observe the molecule phosphine, or PH3. Why PH3? It is a promising sign of life, if detected on another planet. Phosphine is found in Earth’s atmosphere, and its origin is due to human activity or microbes. PH3 reacts easily and effectively with oxygen to create phosphorous acid, destroying the phosphine. Without life on Earth, PH3 would most likely not exist in our atmosphere because of how oxygen-rich the atmosphere is. Thus, an abundance of PH3 in a rocky planet’s atmosphere could very well be suggestive of life. PH3 has been detected in Jupiter and Saturn, in trace amounts, however the mechanism that creates PH3 in these planets occurs deep within the planet where there are very high temperatures and pressures, and then brought up to the surface of those gas giants via convection. Rocky planets have similar environments deep within the interior of the planet, however the rocky surfaces on Mercury, Venus, Earth, and Mars block the PH3 from moving up into the atmosphere where it can be observed.
Venus in particular, also has a very oxygen-rich atmosphere, so any PH3 that could be created via known chemistry would quickly be made into H3PO3. Based on what this science team knew about Venus and phosphine chemistry, they did not expect a detection of phosphine. Thus, when phosphine was detected in 2017, the team decided to do a follow-up observation with the Atacama Large Millimeter/submillimeter Array (ALMA). This telescope would provide a higher sensitivity, as well as better spatial resolution. They could then confirm that another telescope at another time would also detect the presence of phosphine, and map out the location of phosphine in the Venusian atmosphere.
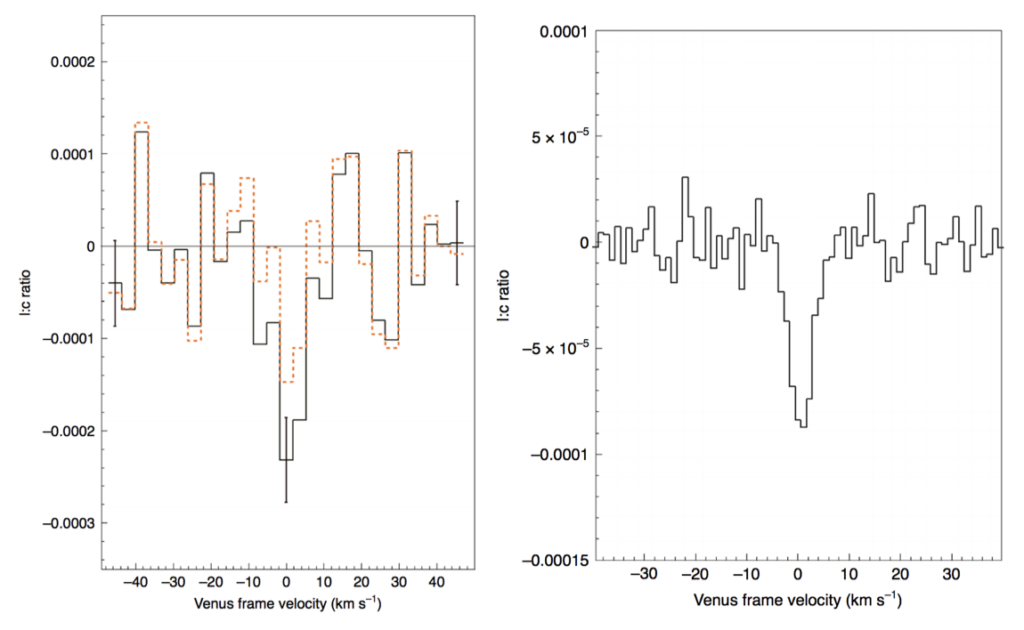
Sure enough, when ALMA gazed towards Venus in 2019, phosphine was yet again detected. The detections from JCMT and ALMA agreed with each other, telling us that not only that this must be a real detection, but over the course of two years the amount of PH3 didn’t seem to change. This team went to great lengths to make absolutely sure that this was a real PH3 detection. They ruled out the possibility of another molecule contaminating the observed line and they had multiple people run the data reduction on the observations. This most certainly means that PH3 has been detected on Venus! Using the spatial resolution that ALMA provides, they also determined that the PH3 must exist at or higher than ~53-61 km into the Venusian atmosphere, and that the distribution of phosphine must be somewhere between completely uniform across the surface and in small distinct patches.
Where did it come from?
Using the flux count from the JCMT observation and a model of Venus’s atmosphere, this team determined that the phosphine abundance is ~20 parts per billion or ppb (for every billion molecules/atoms, 20 of them are PH3). One can also predict the lifetime of PH3 in the atmosphere based on what molecules can destroy PH3 and the mixing within the atmosphere that lofts PH3 to less habitable environments. This team found that the average lifetime of a PH3 molecule is less than 103 seconds. With the combined knowledge of a constant abundance, and the average lifetime of PH3, the production rate of PH3 can then be calculated. They find that PH3 must be produced at a rate of ~106 – 107 mol cm-2 s-1. Now, in order to explain the observed abundance of phosphine, we need to find some way that phosphine is produced at that rate. There has to be some mechanism that produces phosphine whether in the atmosphere, on the surface, in the subsurface, or delivered from space. And this team sought out to model every type of mechanism we know.
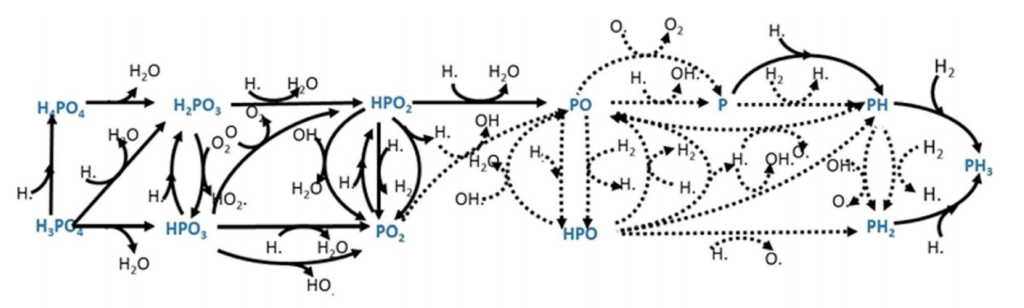
How about known chemical reactions within the atmosphere?
They ran chemical models that utilized ~75 different chemical reactions and thousands of different environmental conditions. None of which even approached their lower limit of PH3 of 10 ppb.
From below the surface of Venus?
They ran simulations of chemistry that occurs within the subsurface, with the idea that the PH3 could be delivered up to the atmosphere, and they found that the oxygen abundance within the crust and mantle of Venus was way too high to support PH3 production and release.
Oooh here’s an idea. Lightning!
While lightning does occur on Venus, and can produce trace amounts of PH3, the team found that lightning didn’t occur often enough and was not efficient at producing PH3. In fact it produced 10,000,000 times less than the amount detected.
How about getting phosphine from meteorites, or one big comet!
Assuming Venus doesn’t get many more meteorites than Earth does, there’s not nearly enough material on meteorites to produce the levels of phosphine detected. And there is no evidence of a large impact in Venus’s recent history.
Volcanos!!
There are volcanoes on Venus, and they can produce phosphine. But to get near the amount that is observed, Venus would have to be 200 times more volcanically active than Earth, and we see no evidence of that.
Aliens?

The phosphine detection corresponds pretty nicely with the Hadley cells within Venus, where temperatures and pressures are very similar to Earth, thus life-friendly. However what is not very life-friendly is the incredibly concentrated droplets of sulfuric acid that no life on Earth has been proven to withstand. The only ‘life’ that could possibly, maybe, survive here are tiny microbes. But life has proven to be adaptive and resilient so….Maybe???
What now?
Here is what we know. Astronomers have detected the molecule PH3 towards Venus’s atmosphere. This is odd because there are a lot of oxygen bearing molecules that would easily destroy PH3 unless there was some source of constant production of PH3 which astronomers cannot identify as of now. This means that there is unknown chemistry happening in the atmosphere of Venus. One solution could be that there are extremophiles that survived Venus’s run-away greenhouse effect from billions of years ago, and exist within a zone of Venus that is mildly habitable. There very well may be other solutions that involve unknown chemical and environmental conditions within Venus. One thing is for sure, you’ll never know if you don’t go.