Title: X-ray photodesorption from water ice in protoplanetary disks and X-ray dominated regions
Authors: R. Dupuy, M. Bertin, G. Fèraud, M. Hassenfratz, X. Michaut, T. Putaud, L. Philippe, P. Jeseck, M. Angelucci, R. Cimino, V. Baglin, C. Romanzin, and J. H. Fillion
First Author’s Institution: LERMA, Sorbonne Universitè, Observatoire de Paris, Universitè PSL, CNRS, Paris, France
Status: Published in Nature [closed access]
So, how are baby stars, X-rays, and planets related?
Surprise, the answer is CHEMISTRY! Planet formation occurs in protoplanetary disks surrounded young baby stars, known as T-Tauri stars. These baby stars, quite like baby humans, are loud and tend to throw temper tantrums in the form of elevated levels of high energy radiation, such as X-rays. Stellar radiation directly impacts the physical disk structure and triggers chemical reactions in the disk through ionization, which causes the destruction of old molecules and formation of new molecules. This process directly impacts the materials available in the formation of planets.
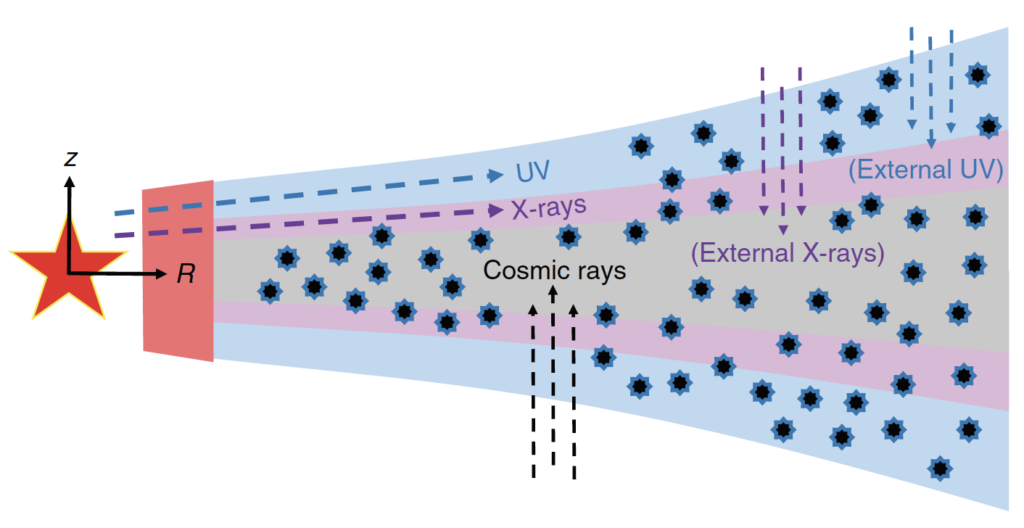
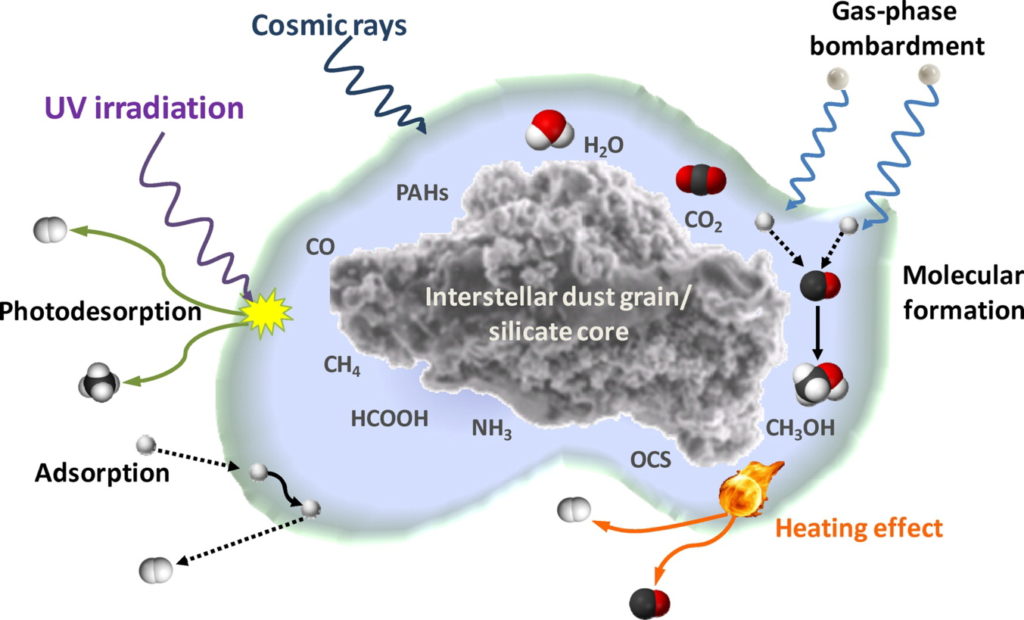
X-rays are able to penetrate deep into the inner disk layers (Figure 1) where ices exist and planet formation occurs. Ices are complex systems (Figure 2) essential in planet formation, as ice provides a sticky coating on dust grains. This coating allows for inelastic collisions that eventually leads to planet formation. However, the desorption of water ice by X-rays had not been studied before this paper. Today’s paper uses experimental techniques to determine if X-rays can desorb water ice via a process known as X-ray photodesorption. Water is a high interest molecule when studying ice chemistry, as water is the main constituent of interstellar ices and is essential for the possible formation of life. However, the desorption of water ice by X-rays has not been studied before.
Science with Space Lasers
X-ray Beams and Vacuum Chambers
The authors use an ultra-high vacuum chamber, where they radiate pure water ice with a SEXTANTS X-ray beam (energies from 520-600 eV). Mass spectrometry is used to monitor the composition of the desorbed gas, and from these data they are able to calculate photodesorption yields, which are the numbers of molecules desorbed per X-ray photon that interacts with the ice (Figure 3). They find that X-ray photodesorption is caused by the following process. X-rays excite an electron from a 1s orbital (the lowest energy orbital) in an oxygen atom from an HO molecule. The excited electron can be ejected from the molecule, a process called the auger effect. The ejected electron can then desorb other chemical species through a transfer of kinetic energy, or the electron can ionize neutral species, thus triggering other chemical reactions.
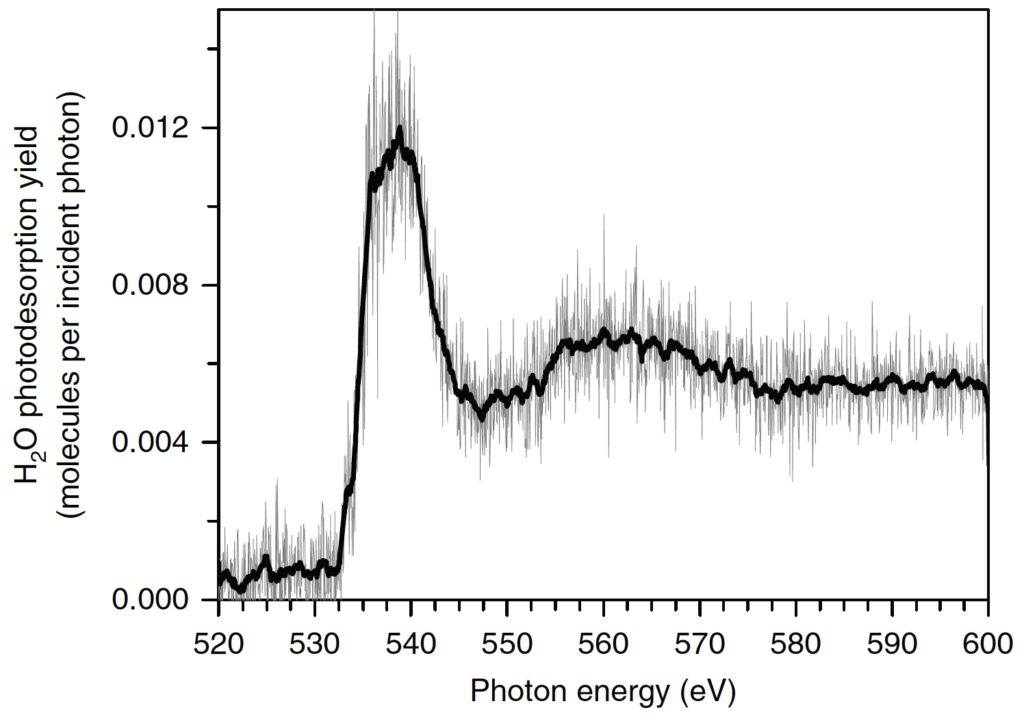
The authors report that the ejected auger electron lacks sufficient energy to both desorb and ionize a species. Meaning, X-rays can indirectly “knock-off” in-tact water molecules from the ice into the gas, while leaving behind broken and reactive species in the ice. This observation is significant, since it suggests that the majority of the HO abundance is conserved, while leaving the more reactive molecules and ions trapped on the ice, where they can potentially go on to form more complex molecules. They arrive at this conclusion by comparing the photodesorption yields of neutral and ionic species, where the photodesorption yields of neutral species (like water) were several orders of magnitude higher than the photodesorption yields of ionic species.
Applications to Models
Our understanding of chemical evolution in astronomical settings is dependent on observations, experimental data, and computational modeling. These three subdivisions feed into each other to provide a complete picture of chemistry in our universe. This paper claims to be the first to consider X-ray photodesorption rates of water ice that can be implemented into chemical models. However, X-ray photodesorption has not been tested in a chemical disk model, so at this time it is still unclear how much of an impact X-rays have on ices (and potentially planet formation and composition) within protoplanetary disks.
As astronomers have been able to observe protoplanetary disks at higher and higher resolution, such as the DSHARP survey, we have begun to realize how complex protoplanetary disk environments are. And with this knowledge, our physical and chemical models of disks are becoming increasingly more complex. This paper opens a whole new door in chemical modeling of protoplanetary disk environments; a door to understanding how X-rays emitted by the central baby star can impact ices and potentially planets.
