Title: N-Graphene Synthesized in Astrochemical Ices
Authors: K K Rahul, M Ambresh, D Sahu, J K Meka, S -L Chou, Y -J Wu, D Gupta, A Das, J -I Lo, B -M Cheng, B N Raja Sekhar, A Bhardwaj, H Hill, P Janardhan, N J Mason, B Sivaraman
First Author’s Institution: Physical Research Laboratory, Navrangpura, Ahmedabad, India
Status: Submitted to ArXiv [Open Access]
Space Dust: The Universe’s Smallest Factory
Let’s be honest. Space dust is cool (literally, it’s about 5 K in the interstellar medium [ISM]). When a molecular cloud collapses to form a star and protoplanetary disk, dust grains eventually collide and stick together to form planets. But, before dust grains become very big planets, first they’re very small chemical factories. In the ISM, dust grains are typically about 0.5 microns in size; for reference, a strand of human hair has a diameter ranging between 17 to 181 microns.
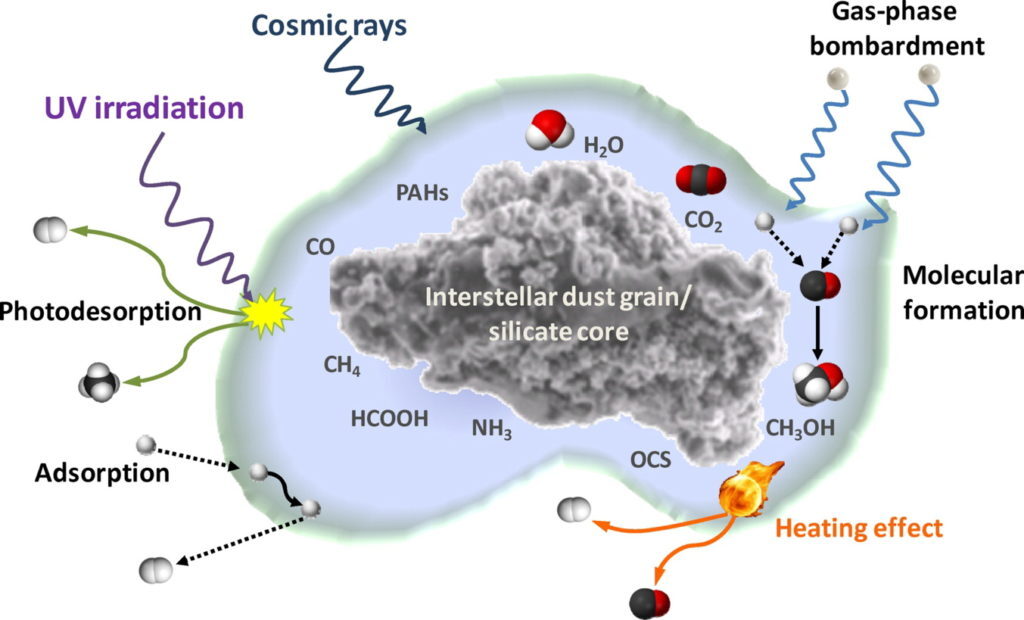
As gas particles collide with dust grains, the particles can stick to them, creating a new icy coating on the grain surface. This layer of ice effectively becomes the smallest operating chemical factory in the Universe, where the majority of molecules are formed in space. Ice chemistry is driven by irradiation from cosmic rays, ultra-violet and infra-red photons present in the ISM. Irradiation can break apart or excite molecules in the ice, thus enabling the “factory” production of even more complex molecules, including molecules significant to the origins of life as we know it (Figure 1).
In recent years, more and more complex molecules have been detected in space, such as benzonitrile. Benzonitrile (Figure 2) is a 6-carbon ring and tied directly to an entire group of molecules known as polycyclic aromatic molecules. Unfortunately, we still don’t know exactly how carbon-based molecules, such as benzonitrile and others, affect the evolution of our icy chemical factories. Today’s paper seeks to determine exactly that: what happens if we irradiate benzonitrile ice?
From Benzonitrile to N-Graphene
The experimental procedure described in today’s paper was performed at the National Synchrotron Radiation Center. A benzonitrile ice film was formed by depositing gaseous benzonitrile into a vacuum chamber at 4 K. The benzonitrile ice was irradiated by 9 eV photons for nine hours, while actively monitoring the ice using VUV and IR spectroscopy. The system was then heated to room temperature (273 K), and the remaining products were imaged using High Resolution — Transmission Electron Microscopy (HR-TEM).
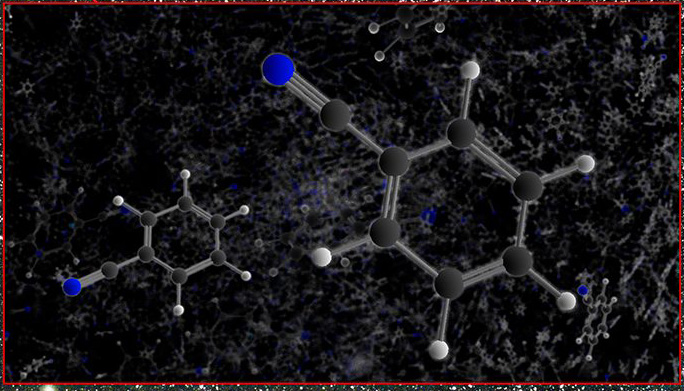
Using HR-TEM, the authors discovered that large sheets composed of hexagonal shapes were left behind after irradiation. This, combined with the IR and VUV spectroscopy, suggested that the irradiated benzonitrile formed a sheet of nitrogen bearing graphene molecules, or N-graphene. Graphite, which you may recognize as pencil lead, is formed when many sheets of graphene overlap. So, imagine this experimental product as a single sheet of pencil lead with a few extra nitrogen atoms thrown in (Figure 3).
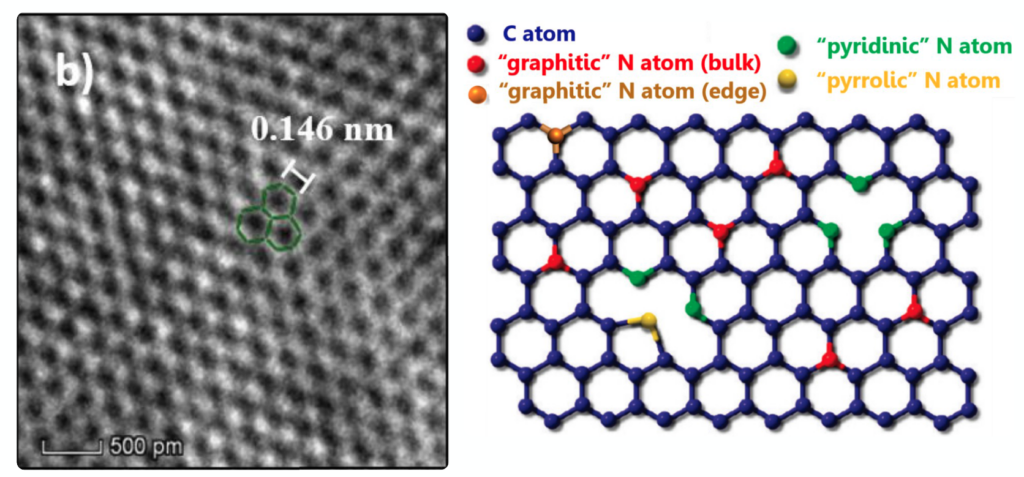
N-Graphene: Protector of Volatiles
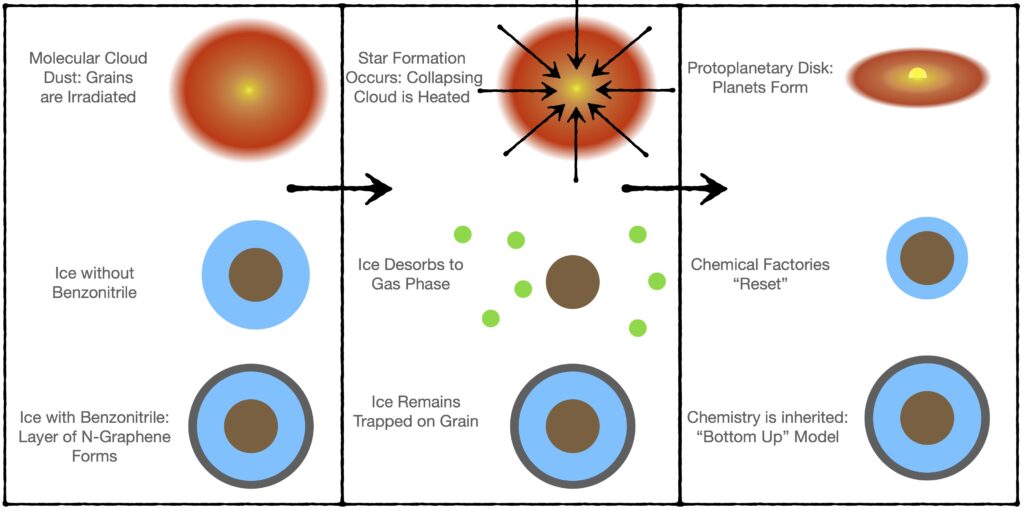
So, why is N-graphene so important in our chemical factories? It turns out, it is really hard to make N-graphene go into the gas phase, just imagine trying to boil pencil lead. In other words, N-graphene is refractory. As a molecular cloud collapses to form a star, the dust grains are heated up. Grains coated in N-graphene will be able to retain their icy layers, but dust grains with smaller molecules, or volatiles such as H2O, CO, or CH3OH, will lose their layers of ice as the system transitions into a planet forming region.
This discovery is significant, because it suggests that N-graphene is able to shield small volatile molecules during the star formation process, thus forcing the volatiles to continue working in the production of more complicated molecules in dust chemical factories. In the absence of refractory molecules, like N-graphene, the volatiles would desorb back into the gas-phase.
Today’s paper shows that benzonitrile, a molecule observed to be in space, can be irradiated to form N-graphene, a refractory molecule. Their experimental results support the theory that the chemical abundances and species available during the planet formation process are inherited from molecules formed during the molecular cloud stage, otherwise known as the bottom-up model.
It’s too bad we can’t reach N-graphene in space the next time we forget a pencil in class.